- October 6, 2016
- No Comment
- 167
Don’t use Homeopathic Teething Tablets & Gels: FDA
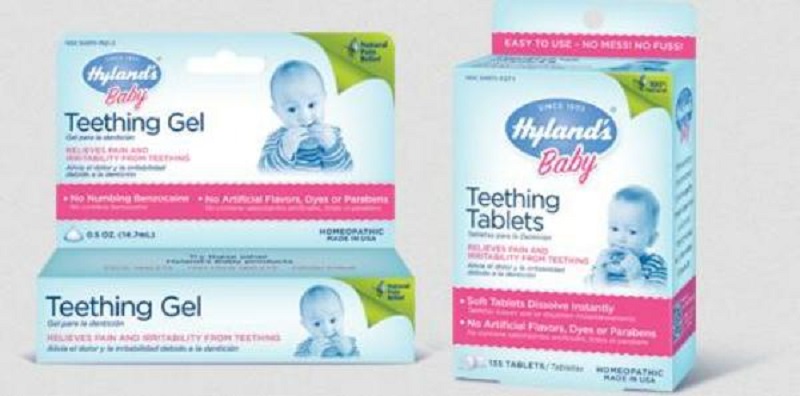
The U.S Food & Drug Administration (FDA) issued a press statement on 30th September 2016 and warned for using the homeopathic teething tablets & gels due it can create a risk to new born babies and children. The FDA released recommendations that consumers should immediately stop using these kinds of products. The CVS, Hyland’s and some others had distributed the alleged Homeopathic teething tablets & gels at retail stores & online. The FDA issued medical care warnings to consumers that if they find breathing problem, excessive sleepiness, seizures, urinating difficulty or muscle weakness after using homeopathic teething tablets or gel, then immediately contact to your medical assistant.
The director of the Center for Drug Evaluation & Research of FDA Janet Woodcock said that “We recommend that parents should keep away for giving homeopathic teething tablets & gels to their children and find advice from their health care professional. The FDA is monitoring & gathering information about the reaction regarding the use of homeopathic teething tablets & gels. The agency has asked from health care professionals & consumers to submit their claims of quality problems after using of homeopathic teething tablets and gels, in order to take stronger decision. It is important that FDA is investigating this issue because homeopathic teething tablets & gels weren’t approved or evaluated by FDA.